Electrochemical study of commercial and synthesized green corrosion inhibitors for N80 steel in acidic liquid†
Abstract
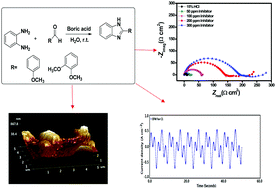
The present paper deals with the investigation of two synthesized benzimidazole derivatives (BZs) as corrosion inhibitors for N80 steel under static conditions in a 15% HCl corrosive environment and in the temperature range of 30 to 90 °C. The corrosion inhibition potential was investigated by electrochemical, gravimetric, and surface screening approaches. BZs are good corrosion inhibitors at 30 °C but weak inhibitors at high temperatures. The values of Gibbs free energy and enthalpy of adsorption suggest that BZs adsorb on the metal surface dominantly via physical interaction. Evidence of BZ adsorption on the N80 steel surface has been provided by scanning electron microscopy (SEM) and atomic force microscopy (AFM). Four different formulations of BZs have been developed using BZs as the base component and additives like potassium iodide, SDS and N acetyl cysteine. The corrosion inhibition performance of the four developed formulations was examined at different experimental temperatures and their inhibition performance was compared with that of a commercial corrosion inhibitor. The results reveal that the BZ based formulations (F4BZ-1 and F4BZ-2) can compete with the commercial inhibitor even at severe temperatures. The obtained inhibition efficiency at 90 °C for a commercial inhibitor is 95.46% and for F4BZ-1 and F4BZ-2, it is 92.94% and 90.61%, respectively.


 Please wait while we load your content...
Please wait while we load your content...