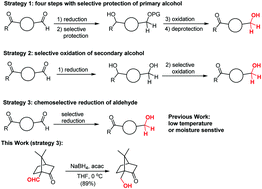
Chemoselective reduction of aldehydes via a combination of NaBH4 and acetylacetone†
Abstract
A bench-stable combination of NaBH4-acetylacetone was developed for the efficient chemoselective reduction of aldehydes in the presence of ketones. This method offers a useful synthetic protocol for distinguishing carbonyl reaction sites, and its synthetic utility is reflected by its moisture tolerance and high efficiency in a variety of complex settings.


 Please wait while we load your content...
Please wait while we load your content...