Micro- and macroscopic observations of the nucleation process and crystal growth of nanosized Cs-pollucite in an organotemplate-free hydrosol
Abstract
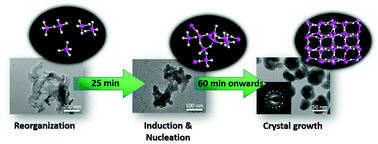
The nucleation and crystal growth of a nanoscale cesium pollucite aluminosilicate zeolite (ANA topology) from an organotemplate-free precursor suspension are reported. By using a new and reactive synthesis recipe (5.5SiO2 : 1Al2O3 : 6Cs2O : 140H2O), zeolite nanocrystals with higher Al content (Si/Al ratio = 2.12) are obtained within 120 min under mild conditions (180 °C), which is much faster and safer as compared to those previously reported. The solid initially experiences amorphous phase re-organization before nucleation, crystallization and crystal growth take place. The resulting Cs-pollucite nanocrystals (average size 55 nm) display a trapezohedron morphology. The nanocrystals are colloidally stabilized in water and they are very active in the base-catalyzed cyanoethylation of dipropylamine reaction, giving 89.6% conversion at 180 °C within 50 min. In addition, a high solid yield of nanocrystals (ca. 70%) is also achieved, thus offering a green pathway for synthesizing zeolite nanocrystals with high basicity on a large scale.


 Please wait while we load your content...
Please wait while we load your content...