Photochemical reactivity of phenyl (methyl-tetrazolyl) ketone – hydrogen atom transfer vs. electron transfer†
Abstract
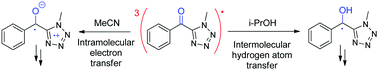
Phenyl (methyl-tetrazolyl) ketone (1) is a synthesis intermediate of tetrazolyloxime fungicides and can also be generated upon their irradiation. Its photolysis is highly solvent-dependent, which prompted us to investigate the reaction mechanism more deeply. The nanosecond laser flash photolysis of 1 yielded the triplet excited state (λmax = 390/570 nm) immediately after the pulse. This was later converted into different secondary species that were identified using their specific reactivity as well as product studies. The ketyl radical (λmax = 315/475 nm) was generated in less than 0.02 μs in a good H-donor solvent such as 2-propanol and in around 0.06 μs in cyclohexane, a medium H-donor solvent. In 2-propanol, ketyl radicals decayed by a second order reaction to yield pinacol (yield 45%); in contrast, in cyclohexane, they decayed by a second order reaction in the bulk, leading to the formation of pinacol (yield 21%), and by recombination with the cyclohexyl radical in the cage in an apparent first order reaction to generate an adduct (yield 10%). In a polar and non H-atom donor solvent such as acetonitrile, the zwitterionic diradical (λmax = 460 nm) was formed in 0.6 μs with the final formation of an atypical dimer. Thus, two mechanisms of hydrogen atom transfer were observed. In the polar acetonitrile solvent, a two-step process occurred, where an electron was transferred first, followed by a proton. In the less polar 2-propanol and non-polar cyclohexane solvents, a one-step process occurred, where an electron and a proton were simultaneously transferred.


 Please wait while we load your content...
Please wait while we load your content...