Synthesis and characterization of hydrazine-appended BODIPY dyes and the related aminomethyl complexes†
Abstract
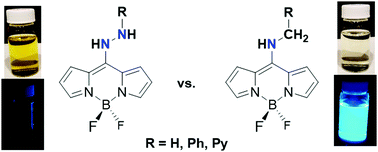
The synthesis and characterization of three new organic hydrazines containing BODIPY dyes is described. The respective aminomethyl complexes were also synthesized to aid in the assignment of the physical properties that were hydrazine-based vs. BODIPY-based. Incorporation of a BODIPY dye into an organic hydrazine introduced a reduction event (average value of −1.70 V vs. Cp2Fe/Cp2Fe+). Although two irreversible oxidation events were observed, it was unclear whether the oxidation events arose from BODIPY-based or amine/hydrazine-based oxidations. The respective BODIPY-appended hydrazine complexes exhibited excited state lifetimes on the order of 2–6 ns, suggesting the presence of a singlet excited state. The excited state lifetimes of the BODIPY-appended hydrazine complexes were about a factor of ten greater than the respective aminomethyl complexes. Computational analysis showed that by appending a BODIPY dye to a hydrazine fragment the hydrazine fragment becomes more susceptible to transfer H2 equivalents as protons and hydrides as opposed to H-atoms, which occurs with common organic hydrazines. Computational analysis also revealed that the BODIPY-based redox events can be used to manipulate the mechanism for H2 transfer from the BODIPY-appended hydrazine, where a BODIPY-based reduction favors H-atom transfer and a BODIPY-based oxidation favors proton transfer followed by hydride transfer.


 Please wait while we load your content...
Please wait while we load your content...