Theoretical study on the ability of bicyclic cryptands to separate alkali-metal isotopes by ion exchange†
Abstract
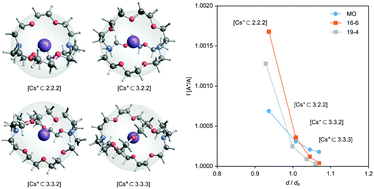
The ability of bicyclic cryptands to separate alkali-metal isotopes was investigated theoretically for the following isotopic pairs: 7Li/6Li, 23Na/22Na, 40K/39K, 87Rb/85Rb, and 137Cs/133Cs. By enclosing alkali-metal cations in cryptands from [1.1.1] to [3.3.3], we optimized the structures of cryptates based on the density functional molecular orbital method and determined the binding potential energies of these cations in cryptates. The ability for isotope separation was evaluated from the reduced partition function of the isotopic pair. Based on the theoretical model that represents the cryptand by a continuum spherical surface with a uniform density, we showed that the reduced partition function increases with a decrease in cryptand size because of the increase in force constant of the cation. Therefore, the smaller cryptand collects heavier isotopes than the cryptand that gives a potential minimum to the cation.


 Please wait while we load your content...
Please wait while we load your content...