Cu/Cu2O/rGO nanocomposites: solid-state self-reduction synthesis and catalytic activity for p-nitrophenol reduction†
Abstract
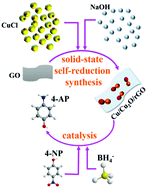
Development of non-noble metal catalysts for the reduction of p-nitrophenol (4-NP) has been the focus of research interest over the last couple of years. Herein, Cu-based nanocomposites supported on reduced graphene oxide (rGO) were successfully synthesized via a solid-state self-reduction, which is a pathway involving cuprous chloride, sodium hydroxide, and graphene oxide. The Cu2O/rGO and Cu/Cu2O/rGO composites were obtained upon heat treatment of the Cu2O/CuO/rGO nanocomposites, which shows that rGO favours the generation of Cu0 species and a high dispersion of Cu-based nanoparticles on the surface. The Cu-based nanocomposites were applied to the catalytic reduction of 4-NP into p-aminophenol (4-AP) in aqueous solution. The results demonstrated that 4-NP was completely converted in 6 min (0.04 mg mL−1 catalyst, 1 mM 4-NP, and 2 mM NaBH4) in the presence of the Cu/Cu2O/rGO nanocomposites. The apparent activation energy of the Cu/Cu2O/rGO nanocomposites for the reduction of 4-NP to 4-AP was calculated as 24.94 kJ mol−1 by using the Arrhenius plot. The good catalytic property can be ascribed to the intense interaction and more electron transfer among Cu0, Cu+, and rGO. This work puts forward a clean and environmentally friendly avenue for the exploitation of supported metal oxide nanocatalysts with outstanding performance.


 Please wait while we load your content...
Please wait while we load your content...