Substrate-switched dual functionalization of alkenes: catalyst-free synthetic route for β-hydroxy and β-keto thioethers†‡
Abstract
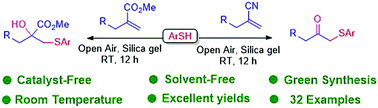
In this study, a substrate-controlled dual functionalization of alkenes under catalyst-free and solvent-free conditions is described. Alkenes possessing different electron-withdrawing groups, namely, ester and nitrile, reacted with a variety of thiols under air to provide β-hydroxy thioethers and β-keto thioethers, respectively, in good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...