Condensation of 9-fluorenone and phenol using an ionic liquid and a mercapto compound synergistic catalyst†
Abstract
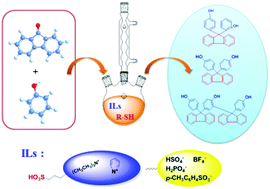
A series of ionic liquids (ILs) were synthesized and their Hammett acidities (H0) were determined using 4-nitroaniline as the indicator. The relationship among IL's structure, the acid strength, and the catalytic performance in the condensation reaction of 9-fluorenone with phenol was discussed. The effective H0 range of ionic liquids that can catalyse the condensation reaction was obtained. Moreover, the catalysis of the mercapto compound co-catalyst was also systematically studied. According to the analysis of how the structure of the sulfydryl co-catalyst affects the percent conversion of 9-fluorenone and the selectivity of BHPF, a mechanism for the reaction in the IL–thiol cooperative catalytic system was proposed. The present work gave a clear clue to design novel IL catalysts for the synthesis of BHPF.


 Please wait while we load your content...
Please wait while we load your content...