Mechanistic insights into n-BuLi mediated phospha-Brook rearrangement†
Abstract
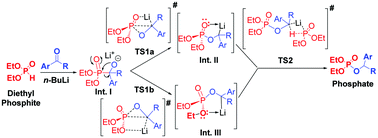
A plausible mechanism for n-BuLi mediated phospha-Brook rearrangement is proposed and substantiated with the help of 31P-NMR and Density Functional Theory (DFT) studies. Based on the 31P-NMR studies, the reaction is proposed to proceed through in situ formation of three lithiated intermediates, which were further supported by the geometry optimized transition states as obtained from DFT studies. Interestingly, DFT calculations revealed the crucial role of the Li-atom in stabilizing cyclic transition states, which in turn resulted in a highly efficient and catalytic phospha-Brook rearrangement even with non-reactive ketones. The calculated rate coefficients using transition state theory also matched well with the experimentally observed facts.


 Please wait while we load your content...
Please wait while we load your content...