The controllable C2 arylation and C3 diazenylation of indoles with aryltriazenes under ambient conditions†
Abstract
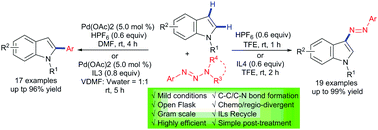
An efficient and regio-divergent method for both the C2 arylation and C3 diazenylation of C2,C3-unsubstituted indoles with aryltriazenes was developed. At room temperature, both reactions were carried out with HPF6/ionic liquids (ILs) as the promoter. The C2 arylation and C3 diazenylation activated by HPF6/ILs show remarkable reactivity, which results in corresponding products with yields of up to 99%. Notably, the practicality of the protocol was further demonstrated via gram-scale operations, late-stage modification and the reusability of the ILs.


 Please wait while we load your content...
Please wait while we load your content...