An ultra-high H2S-resistant gold-based imidazolium ionic liquid catalyst for acetylene hydrochlorination†
Abstract
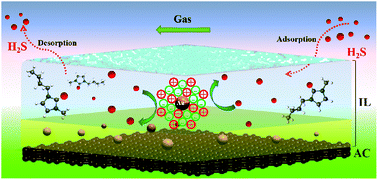
Enhancement of the sulfur resistance of gold-based catalysts is significantly relevant and highly desirable for the development and large-scale applications of these catalysts. In this study, a supported gold-based imidazolium ionic liquid catalyst was shown to be ultra-high resistant towards sulfur poisoning during the acetylene hydrochlorination reaction. To explore the impact of H2S on the as-prepared catalysts, accelerated laboratory-scale poisoning tests were performed, and characterization was achieved by various instruments. The adsorption of H2S on the active sites of Au/AC catalysts, occupying gold species, prevented the reactants, including C2H2 and HCl, from being adsorbed on the catalyst surface. With respect to the Au–IL/AC catalysts, the unique Au–IL colloid complexes, formed from ionic liquids, impart resistance towards H2S; moreover, the ionic liquid layer on the activated carbon surface can prevent the deposition of sulfur impurities on catalysts; in addition, the adsorption of H2S on the ionic liquid layer is a dynamic equilibrium process, and the adsorption equilibrium constant at 180 °C (453 K) is 0.24 L mg−1. The interaction between H2S and the ions in the ionic liquid is weaker than the binding force within ions in the ionic liquid itself.


 Please wait while we load your content...
Please wait while we load your content...