Towards more sustainable synthesis of diketopyrrolopyrroles
Abstract
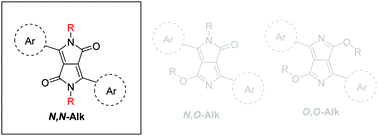
The alkylation of 1,4-diketo-3,6-arylpyrrolo[3,4-c]pyrroles (ArDPP) is one of the most important steps in the synthesis of soluble materials based on these molecules and the polymers derived from them (that are employed widely in putative organic solar cells). Here we report an improvement in their method of synthesis replacing habitual solvent and base. Compared with more usual conditions, we employed acetonitrile as solvent to give higher or similar yields, with less toxic and hazardous waste, lower reaction time and temperature, and allows recycling of unreacted starting materials. Unlike dimethylformamide and N-methylpyrrolidone, which are the most commonly employed solvents. Our reaction conditions have been tested on three different ArDPPs (Ar = thiophene, phenyl and 4-methoxyphenyl) with a variety of linear and branched alkyl reagents. The results show similar and improved results in comparison with the published reports while reducing the waste and hazard of the reaction, as well as simplifying the purification of the products in many cases. Overall this method has lower environmental impact, is more cost effective and requires neither the use of dry solvent nor inert atmosphere.


 Please wait while we load your content...
Please wait while we load your content...