A theoretical study on one-electron redox potentials of organotrifluoroborate anions†
Abstract
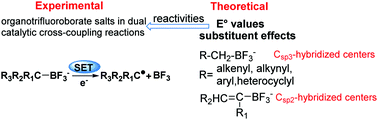
In the dual catalytic Suzuki–Miyaura reactions, the cross-coupling of Csp3-hybridized center organotrifluoroborate salts has been implemented successfully by single-electron transfer (SET), where the redox potentials are important for understanding the reactivities of organotrifluoroborate salts. To this end, the ΔGgas values of 27 organotrifluoroborate anions were calculated by using different composite high-level ab initio and DFT methods. Among different DFT methods, the M05-2X method gave the highest precision with a smallest RMSE of 1.58 kcal mol−1. Then, the 24 E° values of organotrifluoroborate anions were calculated by using the M05-2X method with two types of solvation models including PCM and SMD. It was found that the M05-2X method with a PCM–UAHF model can provide the most accurate results with the lowest RMSE value of 0.16 V. Therefore, the E° values of Csp3-hybridized center organotrifluoroborate anions (R–CH2–BF3−) including R = alkenyl, alkynyl, aryl and heterocyclyl and the Csp2-hybridized center vinyltrifluoroborate anions were systematically investigated with this theoretical protocol. The results indicated that for the Csp3-hybridized center organotrifluoroborate anions, the E° values range from 0.36 V to 1.57 V, and the electron-donating groups (EDG) of –N(CH3)2, –NHCH3etc. can significantly lower the E° values of organotrifluoroborate anions, which are favorable for reactions, whereas the electron-withdrawing groups (EWG) of –NO2, –CN etc. make the organotrifluoroborate anions unfavorable for reactions. For the Csp2-hybridized center organotrifluoroborate anions, the E° values range from 1.38 V to 2.43 V. In addition, the analysis of the natural bond orbital (NBO), the ground-state effect (GE), the radical-state effect (RE) and the energies of frontier orbitals was performed in order to further disclose the essence of the E° value change patterns. The linear relationships between the E° values with substituent constants σ+p and the natural charges of the corresponding C atoms of radicals, etc. were found in different types of organotrifluoroborate anions.


 Please wait while we load your content...
Please wait while we load your content...