Novel styrylpyrazole-glucosides and their dioxolo-bridged doppelgangers: synthesis and cytotoxicity†
Abstract
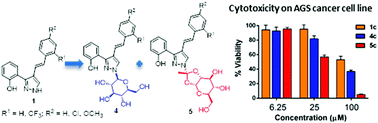
This paper describes the Koenigs–Knorr reaction of (E)-3(5)-(2-hydroxyphenyl)-4-styryl-1H-pyrazoles 1a–d with 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide, using silver carbonate (Ag2CO3) as base and silver triflate (AgOTf) as a promoter/activator in dry dichloromethane. Four per-O-acetylated pyrazole-coupled glucosides 2a–d, the target products of the reaction, were obtained in low yields (11–30%). Surprisingly, the main products of the reaction were new structures with the glucoside forming a dioxolo-bridge with the N-1 of the styrylpyrazole. These compounds 3a–d were obtained in moderate to good yields (39–52%). Compounds 2a–d and 3a–d were deacetylated, in mild conditions, using Amberlite® IRA-400(OH) in methanol, yielding deprotected pyrazole-glucoside conjugates 4a–d and 5b and 5c. The structures of the new compounds were assigned by 1D (1H and 13C) and 2D (COSY, HSQC, HMBC and NOESY) NMR experiments, as well as by MS and HRMS. The cytotoxicity of compounds 1a–d, 4a–d and 5b and 5c against AGS gastric adenocarcinoma cells and MRC-5 lung fibroblasts was evaluated.


 Please wait while we load your content...
Please wait while we load your content...