Anodic fabrication of nanostructured CuxS and CuNiSx thin films and their hydrogen evolution activities in acidic electrolytes†
Abstract
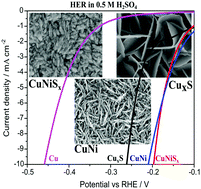
The electrochemical anodization method is advantageous for direct growth of highly ordered and large surface area hybrid nanostructures. Herein, we report the facile electrochemical anodization of copper and copper–nickel electrodes to prepare the respective nanostructured metal sulphide thin films. Vertically aligned 2D nanowall (30–50 nm thickness) like features of mixed-valent CuxS and nanostructures of hybrid Cu–NiSx were grown by controlled galvanostatic deposition (1 mA cm−2). The nanostructured Ni electrodeposits on copper favour the facile anodization and growth of a hybrid mixed-valent CuNiSx thin film. XRD and XPS investigations confirm the presence of mixed-valent Cu and Ni ions as their sulphide thin films. These modified electrodes exhibit pseudocapacitive behavior with a good redox capacitance. The overpotentials required by the Cu–NiSx and CuxS electrodes to attain the benchmark current density of 10 mA cm−2 in 0.5 M H2SO4 are just 195 mV and 270 mV respectively. The hybrid electrode structure (CuNiSx) exhibits improved long-term hydrogen evolution stability (>7 h) compared to the unmodified pristine Cu–Ni electrode (<2 h) in acidic electrolytes. The synergistic effect of porous electrodeposited Ni on the Cu surface and coordinatively unsaturated surface local sites on the hybrid metal sulphides further reduce the overpotential requirement and improve the acidic stability.


 Please wait while we load your content...
Please wait while we load your content...