Synthesis and structural characterization of exemplary silyl triptycenes†
Abstract
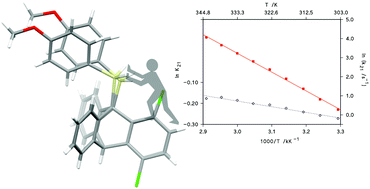
Two novel triptycene derivatives, 9-(p-methoxyphenyl)-silyltriptycene (TRP) and sterically congested 1,4-dichloro-9-(p-methoxyphenyl)-silyltriptycene (TRPCl), are reported. TRP was prepared via both cycloaddition and a more efficient carbanion route. TRPCl, due to sterical congestion, was synthesized via the cycloaddition route. An optimal strategy for the synthesis of sterically congested silyl triptycene derivatives is discussed. The compounds were characterized by NMR, X-ray and PXRD methods. NMR revealed that TRPCl exists in solution in the form of two rotamers: TRPCl-R1 and TRPCl-R2. In TRPCl-R2, the protons in the –SiH2– group are inequivalent and the two corresponding coupling constants 1J(H–Si) differ by 25 Hz (ca. 12%, a high level). From variable temperature NMR spectra, the Arrhenius activation energy for the interconversion of TRPCl-R2 into TRPCl-R1, and free enthalpy and entropy differences between these rotamers were evaluated to be 19.6 ± 0.3 kcal mol−1, 0.54 ± 0.03 kcal mol−1, and 1.23 ± 0.11 cal mol−1 K−1, respectively. Because the entropy difference ΔS21 is very close to R ln 2 ≅ 1.377 cal mol−1 K−1, the entire entropy effect originates from the fact that isomer TRPCl-R2 occurs as a pair of enantiomers, while TRPCl-R1 is a single species. As opposed to the liquid state, PXRD revealed that in the solid state only rotamer TRPCl-R1 is present. X-ray structures evidence high sterical congestion in TRPCl. The presented synthetic approach could facilitate access to more silyl triptycene derivatives, which can be used as building blocks in the construction of more complex and functional molecular scaffolds.


 Please wait while we load your content...
Please wait while we load your content...