Alkyloxy modified pyrene fluorophores with tunable photophysical and crystalline properties†
Abstract
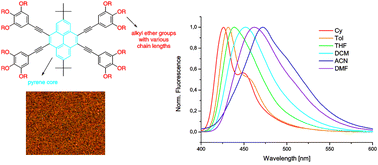
Novel alkyloxy modified 2,7-di-tert-butyl-4,5,9,10-tetra(arylethynyl)pyrenes were prepared through a straightforward Sonogashira coupling approach. Optical properties such as quantum yields and absorption/emission spectra of the fluorophores were investigated by UV/Vis and fluorescence measurements. Aggregation induced excimer formation of the chromophores in polar solvents and in the solid state was proved by the presence of a characteristic bathochromically shifted emission band and a decrease of the emission capability. These results strongly indicate the unexpected observation that the excimer formation of adjacent pyrene rings is not prevented by the introduction of bulky tert-butyl substituents. Single-crystal X-ray and computational analyses reveal the co-planar alignment of adjacent molecules and the presence of π–π-stacking in the molecular packing of the pyrene polyaromatics. Furthermore, fluorescence, DSC and POM measurements indicate that the aggregation behaviour, the thermal characteristics and the crystalline properties are significantly influenced by changing structural features of the attached functional groups at the periphery of the pyrene core.


 Please wait while we load your content...
Please wait while we load your content...