Tunable photophysical properties of thiophene based chromophores: a conjoined experimental and theoretical investigation†
Abstract
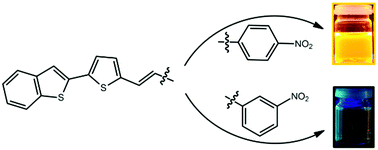
In this paper we report the synthesis and photophysical properties of six novel thiophene derivatives (ThD) with D–π–A structure. The subject of this work is three nitrophenyls (1) BT-Th-NO2 and three benzonitriles (2) BT-Th-CN, substituted at different positions of the aromatic ring ((a) ortho-, (b) meta- or (c) para-). Dyes were obtained using a simple three-step synthesis and their chemical structures were confirmed by 1H and 13C NMR, IR and high resolution mass spectrometry. The influence of positional isomerism on the optical properties has been explored experimentally and theoretically. The photophysical properties were investigated using steady-state and time-resolved spectroscopy and the obtained results were supported by quantum-chemical calculations. TD-DFT calculations indicated that the charge-transfer strength can be correlated with the observed optical properties. In addition, the influence of the acceptor position on the photoluminescence spectra, fluorescence quantum yields and emission lifetimes was specified. We show that the ThD optical properties can be tuned in a wide spectral range by a change made in only a single step of the synthesis reaction.


 Please wait while we load your content...
Please wait while we load your content...