Metal–organic framework derived Co–N-reduced graphene oxide as electrode materials for rechargeable Li–O2 batteries†
Abstract
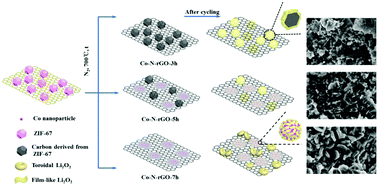
Li–O2 batteries, which involve the reversible formation and decomposition of Li2O2, attract extensive concern for their extremely high theoretical energy density. As main sites for redox reactions, the ability of air electrodes to accommodate discharge products and resist passivation is indispensible for battery performance. Therefore, electrodes with a rational architecture and effective catalysts should be designed to improve the electrochemical performance of Li–O2 batteries. In this paper, Co–N-reduced graphene oxide (rGO) materials are synthesized as catalysts for Li–O2 batteries by simple carbonization of graphene oxide (GO) supported ZIF-67 nanocrystals. The relationship between carbonization time and morphology of the catalysts, and thus affect the electrochemical performance are investigated in order to improve the catalytic activity of the air electrode. With tailored deposition sites and morphology of the discharge products, the air electrode exhibits a significant enhancement in specific capacity and cycling capability. A specific capacity of 3304 mA h gactive materials−1 is acquired for the Co–N-rGO-7 h electrode. By controlling the discharge depth to 500 mA h g−1, the electrode delivers improved performance with stable discharge–charge profiles over 30 cycles.


 Please wait while we load your content...
Please wait while we load your content...