A facile iron-catalyzed dual C–C bond cleavage: an approach towards triarylmethanes†
Abstract
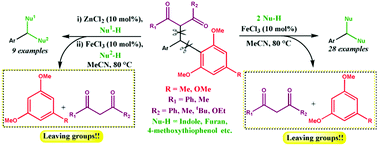
A facile iron-catalyzed dual C–C bond cleaving reaction involving 1,3-dicarbonyl units along with electron-rich and sterically bulky arenes as efficient carbon-based leaving groups has been developed. The scope of the dual C–C bond breaking reaction was studied in detail using a range of nucleophiles to synthesize symmetrical triarylmethanes in good to excellent yields. We found success in isolating the intermediate of the reaction, formed via the initial Csp3–Csp3 bond cleavage, and subsequently converted the intermediate to the final product via the Csp3–Csp2 bond cleavage. A notable difference in the leaving aptitudes of the two carbon-based leaving groups perceived during the study enabled us to develop an application of the dual C–C bond breaking reaction for the synthesis of unsymmetrical triarylmethanes in a stepwise manner. To improve the synthetic utility, we have also fashioned a one-pot procedure to generate unsymmetrical triarylmethanes via sequential C–C bond breaking reactions. In addition to studying a novel dual C–C bond breaking reaction and providing a hint to the mechanistic pathway, the application molded from the study for the synthesis of symmetrical and especially unsymmetrical triarylmethanes may render value to the present work.


 Please wait while we load your content...
Please wait while we load your content...