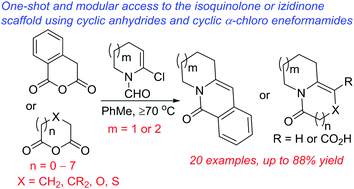
One-shot access to isoquinolone and (hetero)izidinone architectures using cyclic α-chloro eneformamides and cyclic anhydrides†
Abstract
Functionalized izidinones and isoquinolones are commonplace motifs in bioactive molecules. Here, we describe a direct and modular approach to [6,n] and [7,n]-1-azabicyclic unsaturated lactams, most of which bear at least one endocyclic heteroatom. A site-selective hexannulation reaction between cyclic α-chloro eneformamides and cyclic anhydrides is implicated. The scope of the anhydride component in a Castagnoli–Cushman reaction has been extended beyond 5-, 6-, and 7-membered rings.


 Please wait while we load your content...
Please wait while we load your content...