Synthesis of 2-anilinopyridyl linked benzothiazole hydrazones as apoptosis inducing cytotoxic agents†
Abstract
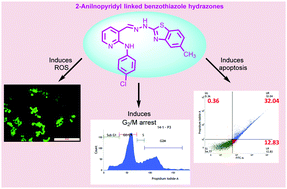
A series of 2-anilinopyridyl linked benzothiazole-hydrazone conjugates (5a-aa) were designed, synthesized and evaluated for their in vitro cytotoxic potency against a panel of cancer cell lines like mouse skin melanoma (B16F10), lung adenocarcinoma (A549), breast adenocarcinoma (MCF-7), triple negative breast cancer (MDA-MB-231) and normal lung epithelial (L132) cell lines. Preliminary screening results revealed that some of these conjugates like 5i and 5l exhibited a significant antiproliferative effect against human breast cancer (MCF-7) with IC50 values of 1.03 and 1.69 μM respectively. Further, detailed biological studies of this promising conjugate (5i) were carried out on MCF-7 cells. The flow cytometric analysis revealed that this conjugate induces cell-cycle arrest in the G2/M phase in a dose dependent manner. Furthermore, in order to determine the effect of the conjugate on cell viability, various cell based assays such as clonogenic assay, ethidium bromide staining, Hoechst staining, detection of ROS generation and annexin V–FITC/PI assays were performed. In these studies, apoptotic features were clearly observed indicating that this conjugate inhibited cell proliferation by apoptosis.


 Please wait while we load your content...
Please wait while we load your content...