Synthesis of dipyrrolopyrazine-based sensitizers with a new π-bridge end-capped donor–acceptor framework for DSSCs: a combined experimental and theoretical investigation†
Abstract
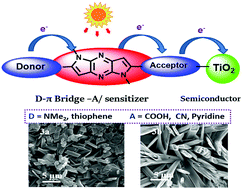
The strategy of developing organic dyes with exceptional optical, thermal, and stable photovoltaic properties has attracted substantial attention in the replacement of high-cost traditional dyes in DSSCs. Herein, we synthesized four dyes with a D–π–A configuration in order to study the variation produced in the optical, thermal, redox and photovoltaic properties by substitution of different donors and acceptors in the dipyrrolopyrazine (DPP) system. These push–pull systems are based on a pyrrolopyrazine spacer, end-capped with donors (N,N-dimethylamine, and thiophene) and acceptors (pyridine, –COOH, and –CN). The multidisciplinary study concerning the optical, thermal, redox, and photovoltaic characterization of the dyes reveals that compound 3a exhibits the best overall conversion efficiency as a sensitizer in TiO2 dye sensitized solar cells. The theoretical calculations together with experimental data allow us to quantify the charge transfer character of dyes.


 Please wait while we load your content...
Please wait while we load your content...