Remarkable isomeric effects on the mechanofluorochromism of tetraphenylethylene-based D–π–A derivatives†
Abstract
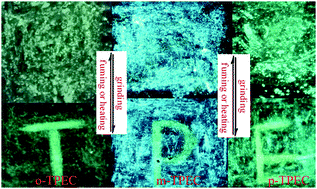
Three tetraphenylethylene (TPE) decorated benzaldehyde isomers were designed and synthesized via the Suzuki–Miyaura coupling reaction with higher yields by changing the linking positions of the peripheral TPE units to demonstrate the isomeric effects on the photophysical and MFC properties. The changes of the linking positions could alter the molecular packing modes, and thus, their solid-state fluorescence properties exhibit remarkable isomeric effects. Interestingly, the three isomers are all aggregation induced emission (AIE) and MFC dyes. And the MFC activities of the three compounds are increased with the sequence of m-TPEC (45 nm) > p-TPEC (39 nm) > o-TPEC (8 nm). X-ray structural analysis, XRD, DSC and theoretical studies suggest that the change from a crystalline phase with a twisted molecular structure to a more planar amorphous phase should be responsible for the MFC properties. These findings suggest that subtle manipulation of the end groups capped at benzaldehyde could significantly alter and tune the photophysical properties. And these results will be of great help in understanding the structure–property relationships of MFC mechanisms and designing more new MFC materials.


 Please wait while we load your content...
Please wait while we load your content...