Glutathione-selective “off–on” fluorescence response by a probe-displaced modified ligand for its detection in biological domains†
Abstract
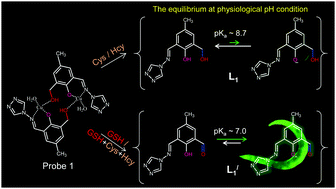
The detection of a specific biothiol in a mixture of biothiols is a challenging task because of their similarity in structure and reactivity. To discriminate between glutathione (GSH) and cysteine (Cys)/homocysteine (Hcy) at physiological pH, the fluorometric “off–on” displacement approach was adopted, utilizing a Cu(II)-complex of a Schiff-base ligand (L1) containing a phenolic aldimine group attached to a triazole moiety along with a dangling benzylic alcohol branch. The weakly fluorescent L1 (quantum yield (ϕF) ∼ 0.006) formed a non-fluorescent 1 : 1 Cu(II)/L1 stoichiometric complex (1) and remained as a dimeric [Cu–L1]22+ species in an aqueous medium. The Cu(II)-centre in 1 is highly susceptible to interactions with the –SH moiety of biothiol to participate in demetallation reactions. Unlike other solvents, the GSH-induced decomplexation of 1 generates strong fluorescence intensity in an aqueous medium, indicating the liberation of the modified L1 (L1′). Experimental evidence suggests that the benzylic alcohol moiety of L1 is itself oxidized to the formyl group during the course of the GSH interaction with 1. However, Cys/Hcy liberates L1 by the partial decomplexation of 1 (∼40%) in the usual displacement pathway to exhibit an intensity increase of less than 5% in comparison to GSH under similar experimental conditions. Unlike L1, there was an acidity increase by about 2.5 pH units for the phenolic-OH of L1′ (pKa ∼ 7.0) with ∼65% phenol to fluorophoric phenolate conversion at pH 7.3, attributed to a large GSH-induced increase in the fluorescence intensity (ϕF = 0.18). It is proposed that GSH induced a comparatively rapid attack at the Cu(II) center in 1, favouring the possibility for radical generation resulting in ligand modification of L1′ along with decomplexation. This is the first report of a biothiol-selective ligand modification exhibiting an “off–on” fluorescence response in the displacement strategy. For biogenic GSH detection and its effect on different biothiol quenchers, in vivo fluorescence imaging was carried out in the multicellular domain on Caenorhabditis elegans (C. elegans), as well as in living human neuroblastoma SH-SY5Y cells. GSH depletion in the presence of a pain killer drug, acetaminophen, was also monitored for C. elegans.


 Please wait while we load your content...
Please wait while we load your content...