2,5-Furandicarboxylic acid as a linker for lanthanide coordination polymers: the role of heteroaromatic π–π stacking and hydrogen bonding†
Abstract
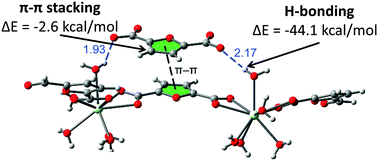
Heteroaromatic carboxylate ligands are very intriguing components of coordination assemblies such as coordination polymers (CPs) due to their inherently multitopic structures. While the fundamental scheme of bonding in CPs is sustained by carboxylic groups, the heteroatoms present in ligands can serve as sites for competitive coordination or can play a sometimes nonobvious role through a range of non-covalent forces such as hydrogen bonding or heteroaromatic π–π stacking. In order to explore the coordination abilities of O-containing heterocyclic ligands, we have assembled five CPs from a heteroaromatic rigid 2,5-furandicarboxylic acid (2,5-H2FDA) linker and lanthanide nodes (Pr, Eu, Ce and Nd) via hydrothermal synthesis: {[Pr2(2,5-FDA)2(H2O)10]n2+·n[(2,5-FDA)2−6(H2O)]} (1), {[Ln2(2,5-FDA)3(H2O)3(DMF)]n·n[(DMF)xH2O]} [where Ln = Pr (2), Eu (3) and Ce (4); x = 2.33 for CPs 2 and 4 and 2.25 for CP 3] and {[Nd2(2,5-FDA)3(H2O)4]n·n[(DMF)1.5H2O]} (5) (2,5-FDA2− = 2,5-furandicarboxylate, DMF = N,N′-dimethylformamide). From the structural viewpoint, CP 1 is a 1D cationic [Pr2(2,5-FDA)2(H2O)10]n2+ coordination polymer having a guest FDA2− counterion stacked in-between coordination chains and six water molecules occupying lattice sites. The 2,5-FDA2− linker exhibits three different coordination modes viz. (μ2-κO,O:κO,O) in CP 1, and unprecedented for this ligand (μ3-κO,O:κO;κO,O) and (μ4-κO:κO:κO:κO) coordination modes in CPs 2–5. The CPs 2–5 are isostructural. Dinuclear [Pr2O18] in CP 1 and tetranuclear [M4O32] [M = Pr (2), Eu (3), Ce (4) and Nd (5)] in CPs 2–5 form secondary building units respectively. Topological analysis reveals that the cationic CP 1 shows 2D-(4,4) rhombohedral grid topology by virtue of H-bonding, whereas CPs 2–5 have bcu topology. The structures adopted by the CPs 1–5 are further explored by Hirshfeld Surface (HS) analysis. In particular, specific properties of heteroaromatic π–π stacking and of hydrogen bonding are evaluated using shape-index and dnorm-mapped HSs, respectively. DFT calculations were employed to identify and quantify contributions to the binding energy of stacked 2,5-FDA2− species in CP 1. It was found that π–π interaction of 2,5-FDA2− moieties made a modest contribution to the formation of the assembly (−2.6 kcal mol−1), which is dominated by the stabilization energy of H-bonds and the electrostatic attraction between counter-ions (−44.1 kcal mol−1 per hydrogen bond). Finally, strong ligand-sensitized Eu3+f–f luminescence is exhibited by CP 3.


 Please wait while we load your content...
Please wait while we load your content...