Nickel-catalyzed cross-coupling of O,N-chelated diarylborinates with aryl chlorides and mesylates†
Abstract
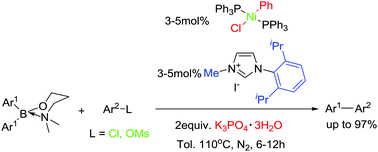
A practical nickel catalyst system consisting of readily available components, trans-NiCl(Ph)(PPh3)2/[Iprmim]I/K3PO4·3H2O in toluene, has been developed for efficient cross-coupling of 3-dimethylaminopropyl diarylborinates with aryl chlorides and mesylates. A small amount of water has been found to be crucial in achieving high efficiency in the system. The nickel catalyst system displayed remarkably higher activities to aryl chlorides and mesylates than the corresponding tosylates in cross-coupling with not only diarylborinates but also diarylborinic acids, arylboronic acids, anhydrides and trifluoroborates as indicated in control experiments. A variety of electronically various biaryls could be obtained in excellent yields by using 3–5 mol% loading of the nickel catalyst while sterically hindered biaryls were obtained in comparably lower yields.


 Please wait while we load your content...
Please wait while we load your content...