A comprehensive spectroscopic and computational investigation on the binding of the anti-asthmatic drug triamcinolone with serum albumin
Abstract
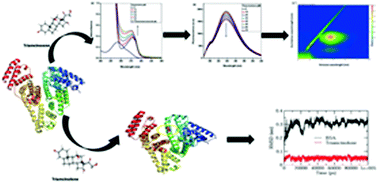
The biomolecular interaction of triamcinolone with bovine serum albumin (BSA) was studied using various multi-spectroscopic techniques in combination with in silico studies. UV-visible absorption, fluorescence spectroscopy and resonance Rayleigh scattering studies confirmed the formation of a BSA–triamcinolone complex. The binding constant was found to be in the order of 103 M−1. Conformational and microenvironmental changes in BSA after addition of triamcinolone were confirmed by circular dichroism and 3D fluorescence spectroscopy, respectively. The negative values of ΔG and ΔH confirmed spontaneous and exothermic binding. The average binding distance between BSA and triamcinolone was also calculated through FRET. Additionally, the effect of metal ions and β-cyclodextrin on the binding of triamcinolone with BSA was also investigated. Molecular docking and site marker displacement experiments unveiled the binding of triamcinolone to BSA at site III located in subdomain IB of BSA. Molecular dynamics simulation showed lower RMSD values and negative total energy, suggesting favourable binding between BSA and triamcinolone.


 Please wait while we load your content...
Please wait while we load your content...