Fluorescent organotin compounds as dyes in silk fibroin (Bombyx mori): ultrasound-assisted synthesis, chemo-optical characterization, cytotoxicity, and confocal fluorescence microscopy†
Abstract
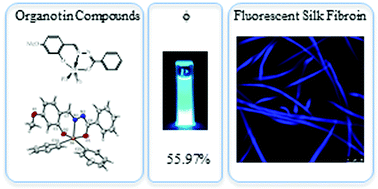
The fluorescent silk fibroin (FSF) is useful in a number of biomedical applications. However, some FSFs are less useful for medical applications because they are derived from toxic compounds. Here we report for the first time a fast (∼20 min), ultrasonic assisted, eco-friendly synthesis of four fluorescent organotin Schiff compounds using the corresponding aldehyde, benzoic hydrazide, and diorganotin oxide (1: MeO-L-SnPh2, 2: tBu2-L-SnPh2, 3: HO-L-SnPh2, and 4: HO-L-SnBu2). These compounds were fully characterized by NMR spectroscopy (1H, 13C, and 119Sn), HRMS, and UV/Vis and fluorescence spectroscopy. Single crystal X-ray diffraction investigations of complexes 1 and 2 are also reported. The as-prepared organotin Schiff compounds exhibit fluorescence at ambient temperature with good photophysical properties (fluorescence quantum yields Φ: 18–55%). These organotin compounds serve as fluorescent dyes in silk fibroin (Bombyx mori). The organotin dyed fluorescent silk fibroin showed a uniform staining, high viability, and good capacity to retain organotin compounds which might be used as innocuous scaffolds in tissue engineering.


 Please wait while we load your content...
Please wait while we load your content...