Conformational preferences of N-acetyl-N′-methylprolineamide in different media: a 1H NMR and theoretical investigation†
Abstract
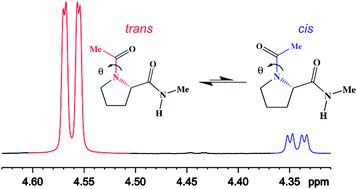
A comprehensive description on the conformational behaviour of small peptide models is of primary importance to elucidate polypeptide and protein structures and folding pathways. In this paper, an extensive conformational investigation of N-acetyl-N′-methylprolineamide (Ac-Pro-NHMe) in both isolated phase and in solution is reported. The joint use of 1H NMR spectroscopy and DFT calculations allowed to determine the most stable conformers of Ac-Pro-NHMe and to evaluate its conformational preferences in different media. Theoretical calculations at the M06-2X/aug-cc-pVTZ level of theory led to the identification of two and six stable conformers in isolated phase and solution, respectively. These geometries reveal the presence of two isomers, where the –NC(O)Me bond can assume a trans (θ = 180°) or cis (θ = 0°) orientation in relation to the –(CO)NHMe group of backbone. The theoretical results show that the trans,trans-IId in a down-puckered γ-turn-like structure and θ = 180°, is the most populated conformer in isolated phase. In polar solvents, like acetonitrile and DMSO, there is a mixture of both polyproline II and α-helix-like conformers, while in water, the trans,trans-IIu, in an up-puckered polyproline II-like geometry, is the less energetic. Therefore, our results show that conformer populations change upon solvent variation, indicating that the conformational equilibrium of Ac-Pro-NHMe is sensitive to solvent effects. The experimental population values, obtained through the integration of the peaks in the 1H NMR spectrum, confirm the change of conformer populations in different solvents. The results reveal that the theoretical level employed in this study is in good agreement with experimental data. QTAIM and NCI analyses combined with orbital-based NBO calculations reveal that the conformers trans,trans-IId and trans,trans-IIu are stabilized by an intramolecular hydrogen bond (IHB) C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯HN in isolated phase. However, these geometries are completely destabilized in solution by the weakening of this interaction. Although IHB is essential to the stability of the two conformers in isolated phase, it represents only a secondary factor to the conformational preferences of the compound. The highest stability of trans,trans-IId in isolated phase can be attributed to hyperconjugative interactions. In solution, an interplay of hyperconjugative, steric and electrostatic effects rules the conformational preferences of Ac-Pro-NHMe.
O⋯HN in isolated phase. However, these geometries are completely destabilized in solution by the weakening of this interaction. Although IHB is essential to the stability of the two conformers in isolated phase, it represents only a secondary factor to the conformational preferences of the compound. The highest stability of trans,trans-IId in isolated phase can be attributed to hyperconjugative interactions. In solution, an interplay of hyperconjugative, steric and electrostatic effects rules the conformational preferences of Ac-Pro-NHMe.


 Please wait while we load your content...
Please wait while we load your content...