Investigation of the selective catalytic reduction of NO with NH3 over the WO3/Ce0.68Zr0.32O2 catalyst: the role of H2O in SO2 inhibition
Abstract
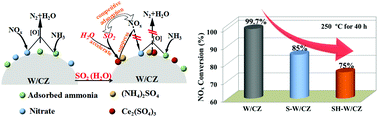
The effect of H2O and SO2 on the selective catalytic reduction of NOx by NH3 (NH3-SCR) over WO3/Ce0.68Zr0.32O2 at 250 °C was systematically investigated using various characterization techniques. NH3-SCR activity revealed that H2O in the reaction gas had little impact on the NOx conversion at 250 °C, whereas, the presence of SO2 slowly decreased the NOx conversion from 99.6% to 85.0% in 40 h. Particularly, the NOx conversion linearly declined to 75% with the co-existence of SO2 and H2O in 16 h. Thus, the presence of H2O obviously accelerated the deactivation of WO3/Ce0.68Zr0.32O2 compared with only the presence of SO2 under NH3-SCR conditions. Nevertheless, the above deactivation resulting from SO2 and H2O could be mostly eliminated by thermal treatment. The characterization via XRD, NO/CO2-TPD, XPS, H2-TPR, OSC and ex/in situ DRIFT demonstrated that (NH4)2SO4 and Ce2(SO4)3 were deposited on the surface of the sulfated catalysts, which decreased the number of active sites and weakened the redox properties of WO3/Ce0.68Zr0.32O2; thus, resulting in the deactivation of the WO3/Ce0.68Zr0.32O2 catalyst. Importantly, the presence of H2O accelerated the generation of sulfates on the surface of WO3/Ce0.68Zr0.32O2, which led to the serious deactivation of the catalyst in the presence of both H2O and SO2. As a result, the Langmuir–Hinshelwood route of NH3-SCR over the catalyst was disrupted due to the shielding of sulfate species.


 Please wait while we load your content...
Please wait while we load your content...