Theoretical investigation of U(i) arene complexes: is the elusive monovalent oxidation state accessible?†
Abstract
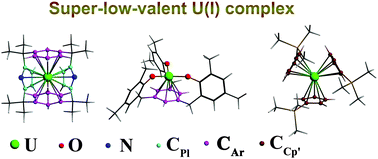
Commonly accepted uranium oxidation states in molecular complexes are III+ to VI+. Recently, this has been extended experimentally by the synthesis of seminal U(II) complexes, Y·[U(Cp′)3] and Y·[URE] (Y = K+(2.2.2-cryptand), Cp′ = C5H4SiMe3, H3RE = (Ad,MeArOH)3mesitylene and Ad = adamantyl). Relativistic density functional theory has been applied to explore whether the uranium oxidation state (+I) is possibly accessible. Calculations show that the U(I) complex of a heterocalix[4]arene (H2L) is energetically stable. It features a 5f5-dominated electronic configuration, four δ(U–Ar)-bond orbitals and a moderate UII/UI reduction potential. Its stability and structural/bonding/energetic properties were corroborated by comparisons with theoretically designed complexes [UI(Cp′)3]2− and [UIRE]2−.


 Please wait while we load your content...
Please wait while we load your content...