Effect of asymmetric modification on perylene derivative molecule self-assembly structures†
Abstract
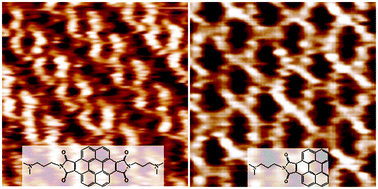
Perylene and coronene derivatives, as family members of the novel discotic mesogenic species and active n-type organic semiconductor molecules, are of interest as modular building blocks for new supramolecular architectures. To understand the self-assembly behavior of these kinds of organic molecules, we have designed and synthesized two derivatives, coronene bisimide bearing dimethylamino-propyl groups (CB-DAP) at two imide positions and perylene with a N-(dimethylamino-propyl)-pyrrole-2,5-dione group (P-DAPPD) at only one of the bay positions, respectively. Two different kinds of long-ranged ordered self-assembled monolayers (SAMs) on highly oriented pyrolytic graphite (HOPG) have been observed for the two types of molecules by scanning tunneling microscopy (STM) at room temperature. A comparative analysis of the STM image features implies how the number and position of intermolecular hydrogen bonding affect achiral and chiral SAM structures of the discotic molecules on the HOPG. It is the asymmetrical inter-substituent interactions that induce the molecular orientation change and further varied hydrogen bonding positions among the molecules adsorbed on the substrate. Density functional theory (DFT) calculation results reveal the formation mechanism of the two different ordered molecular arrays. The design of an asymmetric substituent structure for functional π-conjugated organic systems opens a new avenue for the construction of two-dimensional chiral supramolecular networks with advanced properties.


 Please wait while we load your content...
Please wait while we load your content...