Enzymeless electrochemical determination of hydrogen peroxide at a heteropolyanion-based composite film electrode†
Abstract
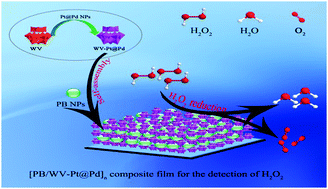
Constructing a specific nanocomposite film with a large surface area and excellent electrocatalytic activity is important for its application as an electrochemical sensor. In this study, for the first time, an ultrahigh performance composite film based on a heteropolyanion PW9V3O403− (abbreviated as WV) decorated by Pt@Pd alloy nanoparticles (Pt@Pd NPs) and Prussian blue nanoparticles (PB NPs) was illustrated via a layer-by-layer self-assembly approach. The composition, morphologies, and nanostructures characteristics of the [PB/WV–Pt@Pd]6 composite film were completely studied by scanning electron microscopy (SEM), atomic force microscopy (AFM), UV-vis spectroscopy, FT-IR spectra, and X-ray photoelectron spectroscopy (XPS), respectively. The special irregular morphology of the [PB/WV–Pt@Pd]6 composite film provides a high specific surface area to boost the electrochemical activity, while the larger roughness and strong electronic cloud facilitate H2O2's fast reduction at pretty low potential. Under optimum conditions, the [PB/WV–Pt@Pd]6 composite film-based sensor presented a broad linear scope of 4.00 × 10−7 M to 2.65 × 10−3 M and a low detection limit of 1.00 × 10−7 M (S/N = 3). Thus, with seeming stability, anti-interference performance, and selectivity, the suggested sensor could be conceivably utilized to detect H2O2 in actual samples.


 Please wait while we load your content...
Please wait while we load your content...