Triazole-amide isosteric pyridine-based supramolecular gelators in metal ion and biothiol sensing with excellent performance in adsorption of heavy metal ions and picric acid from water†
Abstract
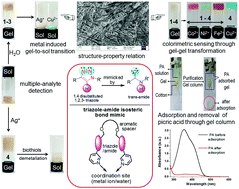
Pyridine-based small molecular gelators 1–4, having a triazole-amide isosteric relationship, have been synthesized. Compounds 1–3 exhibit excellent gelation from DMSO–H2O (1 : 2, v/v), while compound 4 forms a gel in the presence of Ag+ ions in DMSO–H2O (1 : 2, v/v). The change from triazole to isosteric amide has a marked effect on the gelling abilities, minimum gelation concentrations (mgc), thermal stability, mechanical properties, metal ion-responsive character and adsorption properties of the structures, as established by various techniques. All the gels have been successfully applied in sophisticated sensing kits for the selective detection of Cu2+ and Ag+ ions and thiol-containing amino acids. The triazole-based gelators 1 and 3 adsorb heavy metal ions from water with greater efficiency than the isosteric amide-based gelators. The metallogel 4–Ag+ can be used in the efficient removal of picric acid (a nitro explosive) from water.


 Please wait while we load your content...
Please wait while we load your content...