Comprehensive investigations on the action of cationic terthiophene and bithiophene as corrosion inhibitors: experimental and theoretical studies†
Abstract
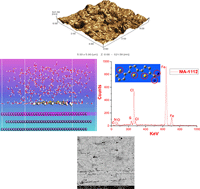
In the present work, the inhibitive action of cationic terthiophene, namely, [2,2′:5′,2′′-terthiophene]-5-amidine (MA-1112), was compared with those of three bithiophene isosteres against corrosion of carbon steel (CS) in 1.0 M HCl. Their inhibition efficiencies (%IE) were assessed by the weight loss method (WL) and electrochemical measurements such as EIS, EFM, and potentiodynamic polarization. The %IE shows a stable performance with an increasing tendency at elevated temperature with exposure time in acid solutions. The adsorption of the investigated derivatives on the CS surface was best described by a Langmuir isotherm and the compounds act as mixed-type inhibitors as evidenced by Tafel polarization experiments. Morphological analysis, via SEM, EDX, AFM analysis, and FTIR spectra manifested significant improvement of the CS surface in inhibited solutions. Computational chemical approaches including quantum-chemical and molecular dynamics simulation methods were applied with insightful outputs consistent with the results from practical experiments. The effect of molecular structure was also discussed in more detail. Compound MA-1112 was demonstrated to be the most efficient inhibitor with %IE around 96% at 18 × 10−6 M. This compound is a modified form of the naturally occurring α-terthienyl compound.


 Please wait while we load your content...
Please wait while we load your content...