A series of new octanuclear Ln8 clusters: magnetic studies reveal a significant cryogenic magnetocaloric effect and slow magnetic relaxation†
Abstract
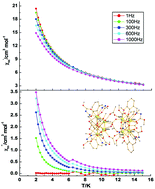
The reaction of a new pentadentate Schiff base hydrazide ligand, (E)-methyl N′-(2-hydroxy-3-methoxybenzylidene)hydrazinecarboxylate (HL), with lanthanide nitrate, and sodium acetate in the presence of triethylamine, resulted in a novel family of octanuclear neutral compounds, [Ln8(μ3-O)4(L)8(CH3COO)4(CO3)2]·xH2O, where Ln = Gd (1), Tb (2), Dy (3), or Ho (4). Compounds 1–4 contained two unusual Ln4 boat-shaped units, which were held by four L− chelating ligands, two μ3-O2−, and one μ4-CO32−. These structures were interconnected by four CH3COO− ions, forming a novel Ln8 cluster. Magnetic studies revealed antiferromagnetic Gd⋯Gd interactions in Gd8, which exhibited a significant magnetocaloric effect and a magnetic entropy change as large as 32.49 J K−1 kg−1 at a field of 7 T at 2 K. In addition, Dy8 exhibited a frequency dependent slow relaxation of magnetization at a zero applied direct current magnetic field.


 Please wait while we load your content...
Please wait while we load your content...