Unravelling the potency of 4,5-diamino-4H-1,2,4 triazole-3-thiol derivatives for kinase inhibition using a rational approach†
Abstract
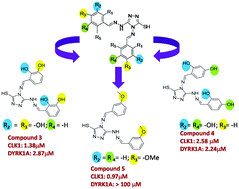
The discovery of potent kinase inhibitors with tunable selectivity and specificity has gained significant impetus in the last few decades. In accordance with this development, herein, we report the design and synthesis of a series of new heterocyclic derivatives comprising a 1,2,4 triazole nucleus, tethered to two flexible linkers on either side, utilizing a simple Schiff base approach (compounds 1–16). The compounds were characterised by various spectroscopic techniques and their biological evaluation was conducted using kinase inhibition assay on two targets, namely CLK1 and DYRK1A. Our investigation reveals that compounds 3–5 bearing hydroxy/methoxy substituents in the aromatic ring display excellent potency towards the receptors in the micromolar range. Indeed, compound 5 bearing a methoxy substitution at the meta position possesses the added advantage of being highly specific towards CLK1 among the targets. To reinforce this observation, we performed molecular modelling studies with the kinase inhibitors hymenialdisine (HMD) cocrystallized with CLK1 and harmine (HA) cocrystallized with DYRK1A kinases as references. We speculate that in either target, the cause of such high potency and selectivity might be attributed to the involvement of flexible linkers on either side of the triazole core, which might have compelled the ligands to enter into the active site of the receptor and maximize non-covalent interactions. Interestingly, we consider the selectivity of compound 5 to be due to the involvement of a H-bond between SH of triazole and Glu 169 carbonyl in the receptor, which is absent in the other derivatives for both targets. Thus, our efforts focus on how the variation/position of substituents in the inhibitors has a profound impact on potency and selectivity. Indeed, this work establishes innovative design principles towards the development of a therapeutic agent for the treatment of neurodegenerative diseases like that of Alzheimers.


 Please wait while we load your content...
Please wait while we load your content...