Contrasting interactions of DNA-intercalating dye acridine orange with hydroxypropyl derivatives of β-cyclodextrin and γ-cyclodextrin hosts†
Abstract
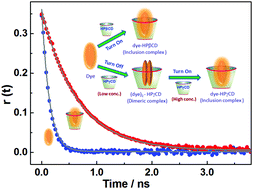
Acridine orange, a well known DNA intercalating dye, exists in two prototropic forms, the cationic form AOH+ and neutral form AO. Systematic investigations on the interactions of AO and AOH+ with two homologous cyclodextrin derivatives, hydroxypropyl-β-cyclodextrin (HPβCD) and hydroxypropyl-γ-cyclodextrin (HPγCD), having different cavity sizes, reveal interesting modulations in the photophysical and acid–base properties of the dye. Ground state absorption, steady-state fluorescence, circular dichroism and time-resolved fluorescence studies have documented quite contrasting changes in the photophysical properties of both AO and AOH+ in their interaction with HPγCD as compared to HPβCD. Results indicate that while 1 : 1 inclusion complexes are only formed using HPβCD, leading to significant fluorescence enhancement for both AO and AOH+, the use of HPγCD on the other hand assists dimerization of both AO and AOH+ at lower HPγCD concentration, resulting in significant fluorescence quenching initially, and subsequently there is a clear conversion of the dimeric dye–host complexes to 1 : 1 dye to host inclusion complexes at higher HPγCD concentration, causing a significant enhancement in fluorescence intensity. To the best of our knowledge this is the first report to document sequential fluorescence quenching and enhancement for both neutral and protonated forms of a dye with gradually increasing host concentration. Both HPβCD and HPγCD hosts cause a significant downward pKa shift for acridine orange dye and the results are in accordance with the stronger binding of the neutral AO form with the studied hosts as compared to the cationic AOH+ form of the dye.


 Please wait while we load your content...
Please wait while we load your content...