High-temperature phase transitions, switchable dielectric behaviors and barocaloric effects in three new organic molecule-based crystals†
Abstract
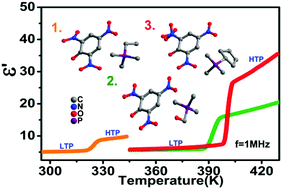
Three new organic molecule-based compounds, ethyl-trimethyl-phosphonium picrate (1, [ETtmp][picrate]), hydroxymethyl-trimethyl-phosphonium picrate (2, [HMtmp][picrate]), and cyclopentyl-trimethyl-phosphonium picrate (3, [CPtmp][picrate]), have been corroborated as high-temperature phase transition materials possessing switchable dielectric behaviors. Compounds 1, 2 and 3 undergo dielectric anomalies which could be tuned in two pronounced dielectric states and switched by reversible phase transitions at 320.8 K, 393.9 K and 398.1 K, respectively. For compounds 1, 2 and 3, not only the phase transition temperatures but also the magnitudes of the dielectric anomalies presented variations by regulating the guest cations. The respective dielectric constants in the high dielectric states are 1.6, 2.7 and 3.6 times those in the low dielectric states for compounds 1, 2 and 3. And considering the big entropy changes (ΔS) in the three title compounds, we predicted the sensitivities of the phase transition temperatures (Tc) to the applied pressure. And the estimated barocaloric coefficients (δTc/δP) in the three title compounds indicate that they potentially perform barocaloric effects of interest for cooling applications under adequate pressure.


 Please wait while we load your content...
Please wait while we load your content...