Insights into the apatite mineralization potential of thermally processed nanocrystalline Ca10−xFex(PO4)6(OH)2†
Abstract
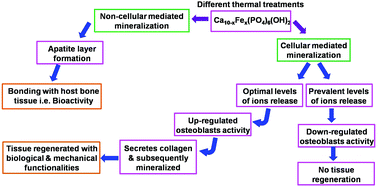
Understanding the crystalline properties of nano-hydroxyapatite (nHAp) from amorphous material is quite a complex process involving investigating thermal treatment, the size and charge of ions and the arrangement of ions or molecules into an ordered lattice, all of which consequently influence structural, morphological and biological properties. Varying the ratio of iron (Fe) incorporated in nano-hydroxyapatite (Fe-nHAp) subjected to different thermal treatments mediated the apatite mineralization potential under non-cellular and cellular conditions, as explored herein. An increase in thermal treatment induces a phase transition from nHAp to β-TCP, which gradually increased due to Fe incorporation and reduced the size aspect ratio followed by the creation of an irregular morphology, as evidenced by XRD and high resolution TEM analyses. The mineralization potential under non-cellular conditions is considerably influenced by the rate of calcium (Ca), phosphate (P) and Fe ionic release. Even if an apatite layer formed by nanocrystalline Fe-nHAp can endow host bone tissue with bioactivity, it is however important to address other biological activity. In line with this, under cellular conditions, the mineralization potentials of Ca, P and Fe ions were assessed using Saos-2 cells in terms of alkaline phosphatase (ALP) activity, gene expression and calcium nodule formation, which show the enhanced potential for mineralization upto a certain ratio of Fe incorporation, as well as under thermal treatment. Thus, it could be perceived that a newly formed bone-like apatite layer is insufficient for the successful use of biomaterials in tissue regeneration, but rather optimal levels of Ca, P and Fe ions are essential to up-regulate osteoblasts, which may promote tissue regeneration.


 Please wait while we load your content...
Please wait while we load your content...