Organometallic acids with azaborine, oxaborine, azaborole and oxaborole scaffolds†
Abstract
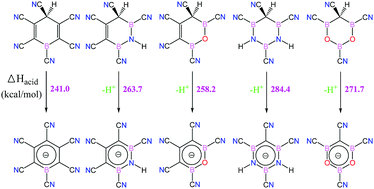
A new class of organometallic acids was designed using azaborole, oxaborole, azaborine, and oxaborine scaffolds. The strategy used in the design of these acids is based on aromatization of the ring after deprotonation and delocalization of the negative charge. Hence, stable anionic conjugated bases were obtained whose conjugate acids were strong acids. The aromaticity of the neutral organometallic acids and their conjugated bases were compared using the NICS index. The enthalpy of deprotonation in the gas phase, ΔHacid, computed employing the B3LYP/6-311++G(d,p) method, was used as an index of acidity. It was found that as the number of boron atoms in the rings increases the acidity and aromaticity decrease. Also, the oxaboroles and oxaborines with BH–O groups were stronger acids than the corresponding azaboroles and azaborines with BH–NH groups. To enhance the acidity, the H atoms of the BH groups were substituted by electron withdrawing groups such as –F and –CN. The –CN groups increase the acidity by about 3–40 kcal mol−1 while the –F substituents do not change the acidity. Also, substitution of the H atoms of CH groups by –CN results in organometallic acids with ΔHacid of 241–309 kcal mol−1 which are stronger than many acids and superacids reported until now. Both C–H and O–H acids, with C and O atoms as proton donor sites, were considered and their acidities were compared.


 Please wait while we load your content...
Please wait while we load your content...