A reusable fluorescent sensor from electrosynthesized water-soluble oligo(1-pyrenesulfonic acid) for effective detection of Fe3+†
Abstract
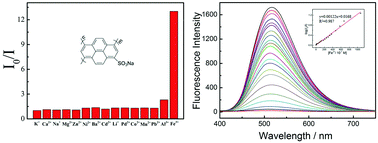
Owing to their excellent fluorescence properties and molecular wire signal amplification effect, water-soluble conjugated oligo-/polymer-based fluorescent sensors have been used for the detection of various metal ions, some of which have already been used in real applications in aqueous environments and biological systems. Herein, we synthesize water-soluble oligo(1-pyrenesulfonic acid) by one-step electropolymerization in boron trifluoride diethyl etherate (BFEE). This oligomer is demonstrated to be an excellent blue light-emitter with a high fluorescence quantum yield of approximately 0.68 in deionized water. We further find that oligo(1-pyrenesulfonic acid) can be employed to effectively detect Fe3+ in aqueous solution due to the aggregation fluorescence quenching of its oligomer chains. Minute sensing analytical results indicate that the oligomer displays good selectivity and a low detection limit (1.00 × 10−7 M), which are better than those of its monomer 1-pyrenesulfonic acid. Moreover, the fluorescence quenching of the oligomer can be recovered by introducing phosphates with outstanding reversibility (97% of its original intensity). With these results, electrosynthesized oligo(1-pyrenesulfonic acid) will be a good candidate for the determination of Fe3+ in aqueous solution.


 Please wait while we load your content...
Please wait while we load your content...