A new fluorescent–colorimetric chemosensor for cobalt(ii) ions based on bis-benzimidazolium salt with three anthraquinone groups†
Abstract
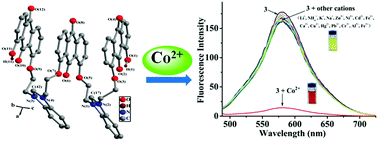
A novel fluorescent–colorimetric chemosensor 1,8-bis{2-{N-[2′-(8′-hydroxy-9,10-anthraquinon-1-yloxy)ethyl]benzimidazoliumyl}ethoxy}-9′,10′-anthraquinone hexafluorophosphate (3) was prepared and characterized by 1H and 13C NMR spectroscopies; its single crystal structure was also obtained. By fluorescence and UV titrations, 1H NMR titrations, and HRMS and IR spectra, we explored the cation recognition performance of 3, and the results revealed that 3 was a highly effective chemosensor for Co2+. After mixing with Co2+, a distinct color change (from orange to red under visible light) was visible to naked eyes accompanied with remarkable fluorescence quenching. A hypochromatic shift (ca. 27 nm) and a new peak at 487 nm were observed in the absorption spectra of 3. Furthermore, 3 was also applied for the detection of Co2+ by using a smartphone with the calculated limit of detection down to 0.47 μM.


 Please wait while we load your content...
Please wait while we load your content...