Simple preparation of carbon–bimetal oxide nanospinels for high-performance bifunctional oxygen electrocatalysts†
Abstract
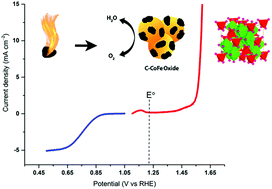
The lack of efficient cost-effective electrocatalysts for reversible oxidation of water is by far the most notorious obstacle in the development of fuel cells and electrolyzers. Here, oxygen bifunctional electrocatalysts based on C–CoFe and C–NiFe oxide nanospinels are developed by simple autocombustion between ethylene glycol/acetate and the metal nitrates. The effects of electronic modulation and the mass (or surface area) effect were examined based on the cyclic voltammograms of the unary and binary metal oxides in alkaline solution, and their high oxygen evolution and reduction activities were attributed to the synergic intermetallic interactions. The C–CoFe oxide, in particular, shows an oxygen evolution overpotential of 350 mV (without iR correction) at 10 mA cm−2 with excellent stability over 10 hours and a Tafel slope of 49 mV per decade. Furthermore, it exhibits the highest oxygen reduction activity among the synthesized electrocatalysts due the particular synergy between Co and Fe centers.


 Please wait while we load your content...
Please wait while we load your content...