Colorimetric and optical Hg(ii) ion sensor developed with conjugates of M13-bacteriophage and silver nanoparticles†
Abstract
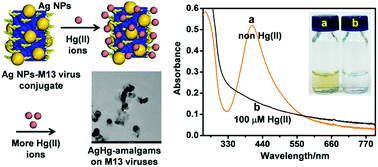
Monitoring heavy metal ions in water is important for human health, molecular biology, and environmental safety. Engineered biological molecules conjugated with nanomaterials are excellent probes for the development of desirable biosensors. Here, we report the fabrication of nanomaterials conjugated with biomolecules (NCBs) consisting of 4E-peptide-engineered M13-bacteriophage (M134E) and silicate sol–gel matrix (SSG)-stabilized Ag nanoparticles. The conjugates are named as SSG–Ag4E NCBs. We have shown that SSG–Ag4E NCBs can effectively pre-concentrate Hg(II) ions through the chemical interaction between the conjugated Ag nanoparticles and Hg(II) ions. With the growth of AgHg-amalgam crystals, the quenching of the SPR from the Ag nanoparticle's absorption band was observed. The amalgam was characterized by XPS, TEM, STEM-HAADF, and STEM-EDS analyses. The sensing performance of the SSG–Ag4E NCBs toward Hg(II) ions was examined and compared with the results from the use of only SSG–Ag NPs and wild-type M13-bacteriophages (M13wild) conjugated with Ag NPs (SSG–Agwild NCBs). As a result, the limit of detection from the SSG–Ag4E NCBs was the best at 10.8 nM. Taken together, we provide evidence that the optimized combination of nanomaterials and bacteriophage could offer a convenient way to develop an effective metal ion sensor.


 Please wait while we load your content...
Please wait while we load your content...