A molecular roundabout: triple cyclically arranged hydrogen bonds in light of experiment and theory†
Abstract
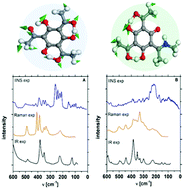
The paper dwells on the synthesis and diverse studies of cyclically arranged hydrogen bridges in tris-hydroxy aryl Schiff bases. Experimental (IINS, IR, Raman and X-ray) and theoretical (CPMD, DFPT and DFT) studies of tris-hydroxy aryl Schiff bases have been performed in the solid state. This is the pioneer spectroscopic investigation of mutual interactions between hydrogen bridges in a cyclic arrangement. The spectroscopic studies have been carried out by IR, Raman and IINS experimental methods and supported by density functional theory (DFT), plane-wave periodic theory (DFPT) and Car–Parrinello Molecular Dynamics (CPMD) calculations. A study of the impact of steric squeezing on the strength of hydrogen bonds is presented. The remarkable dynamics of the bridged protons in the studied Short Strong Hydrogen Bond (SSHB) has been investigated on the basis of first-principles CPMD calculations. The fundamental investigations of the hydrogen bridge vibrations have been accomplished on the basis of isotopic substitutions (NH → ND and OH → OD) and vibrational Potential Energy Distribution (PED) analysis.


 Please wait while we load your content...
Please wait while we load your content...