Combining organocatalysis with photoorganocatalysis: photocatalytic hydroacylation of asymmetric organocatalytic Michael addition products†
Abstract
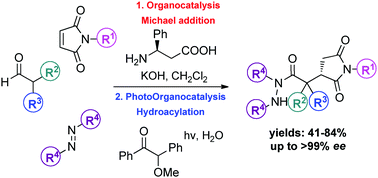
Organocatalysis and photoorganocatalysis are two areas of synthetic methodology that have found wide applications in organic synthesis. Herein, we report a combination of these two stategies, taking advantage of an organocatalytic Michael addition of α,α-disubstituted aldehydes to maleimides as the first step and a photocatalytic hydroacylation of diisopropyl azodicarboxylate as the second step. Employing an amino acid as the organocatalyst for the asymmetric organocatalytic part and an organic molecule as the photocatalyst, the combination of these two strategies led to the desired products. A number of alkyl- and aryl-substituted maleimides were successfully employed, while the protocol can be used on α,α-disubstituted aldehydes leading to products in moderate to high yields (44–84%) and excellent enantioselectivities (98–100% ee).


 Please wait while we load your content...
Please wait while we load your content...