Synthesis, luminescence and magnetic properties of tetranuclear lanthanide-based (Eu4, Gd4 and Dy4) clusters†
Abstract
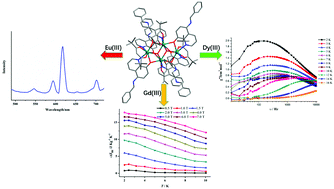
Three analogous lanthanide-containing complexes were isolated, with the molecular formula [Ln4(μ3-OH)2(tmhd)4L6]·2CH3CN (where Ln = Eu(1), Gd(2) and Dy(3); HL = 5-phenyl-8-hydroxylquinoline; and Htmhd = 2,2,6,6-tetramethyl-3,5-heptanedione). Single-crystal X-ray diffraction measurements performed on 1–3 revealed that they were all composed of [Ln4(μ3-OH)2] centers with butterfly-shaped arrangements. Investigations into the luminescence properties and lifetime of 1 showed characteristic emission peaks of the Eu3+ ion and an average luminescence lifetime of 9.26 μs. Detailed magnetic studies suggested that antiferromagnetic interactions existed in 2 and 3, and that there was a remarkable magnetocaloric effect with a −ΔSm value of 17.94 J K−1 kg−1 for 2, and a slow magnetic relaxation process with an energy barrier ΔE/kB of 35.8 K for 3.


 Please wait while we load your content...
Please wait while we load your content...